Convex Parabolic Mirrors
- Subject:
- Physical Science
- Physics
- Material Type:
- Lesson
- Provider:
- Khan Academy
- Author:
- Sal Khan
- Date Added:
- 09/22/2013

Convex Parabolic Mirrors

Students act as mining engineers and simulate ore mining production by using chocolate chip cookies. They focus on the cost-benefit analysis of the chocolate ore production throughout the simulation, which helps them understand the cost of production. As students “mine” with tools such as paperclips and toothpicks, they keep records of their costs—land (cookie), equipment used, cookie size before and after production, and time spent. While the goal is to make as much profit as possible, other costs and goals are taken into consideration—as in real-world mining engineering. For example, mining engineers also consider the resulting amount of destruction to the lithosphere when deciding the best method to obtain ore. Thus, a line item for land reclamation cost is included from the beginning. A provided worksheet serves as a profit and loss statement.

There has been debate about whether the periodic table's newest element should have the symbol Cp or Cn? This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.

Here's a new video about copper, including a nice demonstration and some stories from The Professor. This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.

A copper sulfate reaction filmed with a high-speed camera.
Correcting the time difference calculation by taking into account that there is no year 0
Correction to "Total Final Velocity for Projectile" Video

Cosmic Background Radiation
Cosmic Background Radiation 2 - Redshift of the Cosmic Background Radiation
Cosmological Time Scale 1
Cosmological Time Scale 2
In this video Paul Andersen explains how we can use Coulomb's law to predict the structure of atoms. These predictions can be verified through the use of Photoelectron Spectroscopy (PES). Electron's are help around the nucleus because of differences in charge.
The students discover the basics of heat transfer in this activity by constructing a constant pressure calorimeter to determine the heat of solution of potassium chloride in water. They first predict the amount of heat consumed by the reaction using analytical techniques. Then they calculate the specific heat of water using tabulated data, and use this information to predict the temperature change. Next, the students will design and build a calorimeter and then determine its specific heat. After determining the predicted heat lost to the device, students will test the heat of solution. The heat given off by the reaction can be calculated from the change in temperature of the water using an equation of heat transfer. They will compare this with the value they predicted with their calculations, and then finish by discussing the error and its sources, and identifying how to improve their design to minimize these errors.
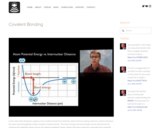
In this video Paul Andersen explains how covalent bonds form between atoms that are sharing electrons. Atoms that have the same electronegativity create nonpolar covalent bonds. The bond energy and bond length can be determined by graphing the potential energy versus the distance between atoms. Atoms that share electrons unequally form nonpolar covalent bonds.
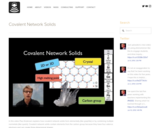
In this video Paul Andersen explains how covalent network solids form elementally (like graphite) or by combining multiple nonmetals (like quartz). Covalent network solids contain elements from the carbon group because they have four valence electrons and can create three-dimensional shapes.

The Professor demonstrates the reactivity of Chromium Trioxide and the department smoke alarms!
Students learn about the physical force of linear momentum movement in a straight line by investigating collisions. They learn an equation that engineers use to describe momentum. Students also investigate the psychological phenomenon of momentum; they see how the "big mo" of the bandwagon effect contributes to the development of fads and manias, and how modern technology and mass media accelerate and intensify the effect.
Learn the physics behind how quadrotors fly and find out how they can by themselves without human help.
Students are introduced to servos and the flex sensor as they create simple, one-jointed, finger robots controlled by Arduino. Servos are motors with feedback and are extensively used in industrial and consumer applications—from large industrial car-manufacturing robots that use servos to hold heavy metal and precisely weld components together, to prosthetic hands that rely on servos to provide fine motor control. Students use Arduino microcontrollers and flex sensors to read finger flexes, which they process to send angle information to the servos. Students create working circuits; use the constrain, map and smoothing commands; learn what is meant by library and abstraction in a coding context; and may even combine team finger designs to create a complete prosthetic hand of bendable fingers.

Students create silver nanoparticles using a chemical process; however, since these particles are not observable to the naked eye, they use empirical evidence and reasoning to discover them. Students first look for evidence of a chemical reaction by mixing various solutions and observing any reactions that may occur. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Students iterate on their initial process and test to see if they can improve the manufacturing process of silver nanoparticles.