Amine Naming 2
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Lesson
- Provider:
- Khan Academy
- Author:
- Khan Academy
- Date Added:
- 02/14/2017

Amine Naming 2
Amine Naming Introduction

Antimony is element number 51 and its chemical symbol is Sb. This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.

Aqua Regia is a special combination of two acids which can dissolve gold.

Argon, an inert noble gas, is element number 18. This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.
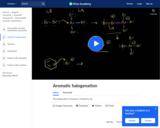
The halogenation of benzene
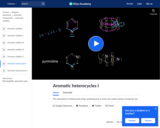
The aromaticity of pyridine and pyrimidine.
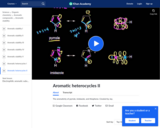
The aromaticity of pyrrole, imidazole, and thiophene.
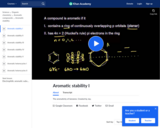
The aromaticity of benzene

The video resource "Aromatics and Cyclic Compounds - Crash Course Chemistry #42" is included in the "Chemistry" course from the resources series of "Crash Course". Crash Course is a educational video series from John and Hank Green.

Arsenic is element number 33. This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.

We discuss the special properties of aspirin and make a sample of it.

Because of its radioactivity and short half life, Astatine is an element few chemists come into contact with. This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.

The video resource "Atomic Hook-Ups - Types of Chemical Bonds: Crash Course Chemistry #22" is included in the "Chemistry" course from the resources series of "Crash Course". Crash Course is a educational video series from John and Hank Green.
In this video Paul Andersen explains how the atomic model has changed over time. A model is simply a theoretical construct of phenomenon and so when we receive new data we may have to refine our model. Ionization energy data resulted in the formation of a quantum model that more accurately reflected the atom.
Mr. Andersen describes atomic structure and tours the periodic table.

Barium meals (barium sulfate, not toxic barium) are among the topics in our updated video about the element Barium. This video is one of the 118 clips included in the periodic table of elements themed collection created by Brady Haran and the University of Nottingham in the UK.
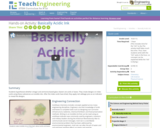
Students hypothesize whether vinegar and ammonia-based glass cleaner are acids or bases. They create designs on index cards using these substances as invisible inks. After the index cards have dried, they apply red cabbage juice as an indicator to reveal the designs.
Students learn the basics of acid/base chemistry in a fun, interactive way by studying instances of acid/base chemistry found in popular films such as Harry Potter and the Prisoner of Azkaban and National Treasure. Students learn what acids, bases and indicators are and how they can be used, including invisible ink. They also learn how engineers use acids and bases every day to better our quality of life. Students' interest is piqued by the use of popular culture in the classroom.
These Pre-Chemistry online modules are designed to function as chemistry preparation for first year chemistry students. It is particularly useful for students who, for various reasons, are otherwise not confident in their preparation for first year university level chemistry. However, the module can be used as a practical and valuable review for all students. The module focuses on the development of fundamental numeracy and problem solving skills that are widely applicable to students in a variety of first year chemistry courses including those directed to students in life science, engineering and natural and physical sciences. These modules function effectively in both online, hybrid or even as preparation for entirely traditionally delivered courses.